Aluminum Toxicity
- Type
- Nutrition
- Roots
- Stunted, Poor Nodulation, Necrotic
- Plant Size
- Stunted
- Field Distribution
- Uniform, Low Areas, High Areas
- Season
- Early Vegetative, Mid To Late Vegetative, Flowering, Pods Present
- Cropping System
- Soybean Followed By Soybean, Conventional Till, Reduced Till
Introduction
Aluminum is a chemical element that causes severe toxicity to plants. In North Carolina, the soil test report does not provide information about the aluminum concentration in the soil. However, as aluminum occurs only in low pH soils, the pH information can be used to predict a potential aluminum toxicity. The pH level and exchangeable acidity information present in the soil test report are used to calculate the lime requirement to raise the soil pH and neutralize the toxic aluminum.
Occurrence
Aluminum (Al) toxicity occurs in low pH soils. When the pH is lower than 5.5, except for mineral-organic and organic soils, Al toxicity may be observed.
Symptoms
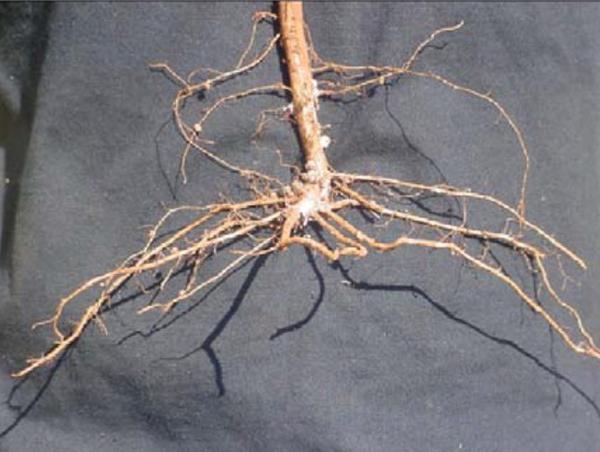
Plant growth is severely stunted. Deformed root tips, overall lack of root development (Figure 1), and no or poor nodulation will be seen (Figure 2). Plant tissue analysis may reveal low or deficient phosphorus (P), calcium (Ca), and magnesium (Mg) levels. Plant micronutrient levels may be high or excessive.
Management
The visual toxicity diagnosis needs to be confirmed by diagnostic soil analysis. Procedures for diagnostic sampling can be found at NC State Extension’s soil fertility webpage. When Al toxicity is detected in a soybean field during the growing season, recovery of the current crop is unlikely. Liming at an appropriate rate based on soil testing should reduce the problem in future crops.