Phytophthora Crown Rot
- Scientific Name(s)
- Phytophthora cactorum
- Type
- Disease
- Leaf Condition
- Tip burn, Marginal necrosis, Interveinal necrosis, Complete necrosis, Stunted
- Leaf Location
- Entire leaf, Young, Mature
- Roots
- Stunted, Necrotic
- Crown
- Crown necrotic, Internal discoloration
- Petioles Condition
- Death, Collapse
- Plant Size
- Stunted
- Field Distribution
- Random, Low areas
- Prior Environmental
- Rain, Temp. below 15°, Temp. between 20° and 32°
- Season
- Post transplant, Early spring, Pre harvest, Harvest
- Cropping System
- Annual plasticulture, Perennial matted row
Introduction
Strawberry infection by Phytophthora cactorum occurs on poorly drained, over irrigated soils, or during long periods of rain in warm climates. Symptoms of disease are enhanced during periods of high water need, such as after transplants are set, during hot dry weather, or as the fruit load increases. The pathogen has become very important in the last 20-25 years (1999-2024).
Symptoms and Signs
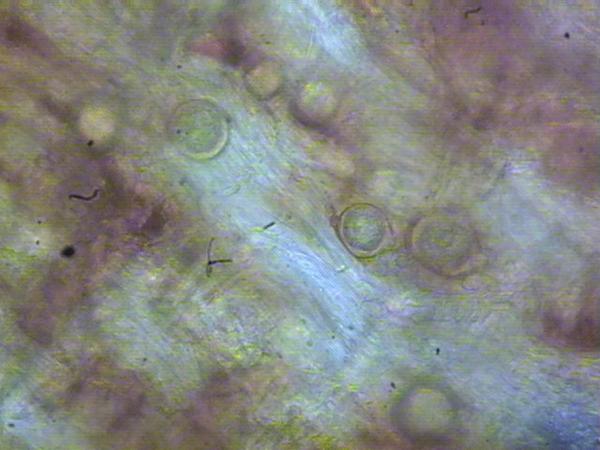
The most diagnostic feature of plants with advanced symptoms is a collapse of plants and a deep dark red discoloration of the crown (Figure SS-1). Stunting of plants or wilting of young leaves are the first symptoms and may appear at any time during the season. Infected plants may remain stunted, or foliage may turn bluish and the entire plant may wilt rapidly until total collapse. Wilting is often accompanied by “drought” symptoms on the leaves such as browning of leaf margins often progressing between the veins and a sharp line between damaged and healthy tissue (Figure SS-2). Plants may break freely at the upper part of the crown when pulled for sampling. Plants may occur in low lying areas and then are often clustered, or they may appear more in a scattered pattern, especially if the disease came on infected plants (Figure SS-3). When the crown is first infected, a longitudinal section reveals watersoaked and light brown tissue, but, as disease progresses, extensive necrosis appears that is uniformly brown and not restricted to the vascular tissue (Figure SS-1; Figure SS-2). The dark brown discoloration may appear at the base, middle or top of the crown. Roots attached to the affected crown area are frequently black at the point of attachment and commonly have diagnostic oospores in the root tissue (see below; Figure SS-4). Root systems are also frequently discolored and have a low level of fibrous (secondary and tertiary) roots. In contrast, crown tissue infected by the anthracose pathogen (anthracnose crown rot) takes on a darker cinnamon color, is more firm, often has a “marbled” appearance and roots tend to remain white to brown at the point of attachment. Also, the root system tends to remain fibrous with a healthy white appearance.
The disease can appear in plug production facilities when introduced on infected tips or through contaminated water sources. Tips fail to root, the roots turn black, and the lower petioles of the leaves turn dark brown to black (Figure SS-5). Plug plants will die or remain stunted especially once frequent watering stops.
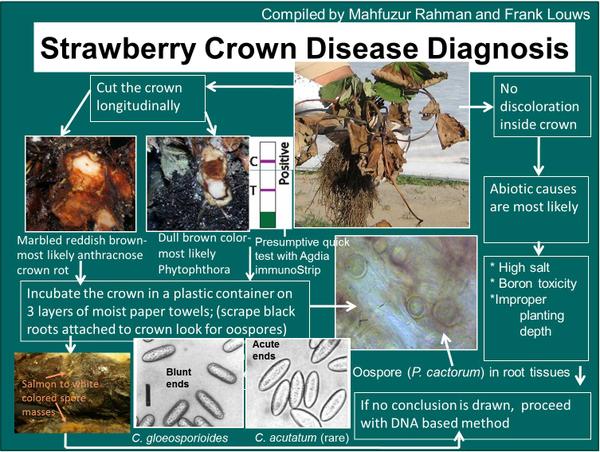
Phytophthora crown rot may be confused with anthracnose crown rot. A series of different symptoms and diagnostic steps can be taken to discern between the two crown rot problems and these are highlighted in Figure DP-1.
Disease Cycle
Infection is favored by wet conditions. The primary inoculum sources include oospores in the soil and infected transplants. Once introduced, the pathogen can survive for many years on some farms whereas other farms do not have repeated problems year after year. Oospores produce zoospores that infect primarily through wounds in stolon stumps, rhizomes, or freshly dug runners. Frigo plants, or plants from cold storage, are extremely susceptible to crown rot. Disease expression is influenced by time of planting and environmental conditions. Since the fungus requires warm temperatures and prolonged wetness, initial plant collapse can occur in the Fall within 1 month after planting with additional plant collapse occurring the following spring as regrowth occurs and especially as fruit develop. The pathogen can move with water flow patterns in the field and in plug production facilities.
Management
Cultural
1. Site Selection and Preparation
Choose a site with adequate soil drainage and avoid fields with a history of disease. Plant in raised beds, not low wet spots, and rip fields during preparation to break hardpans and improve drainage. Soil fumigation may help to reduce inoculum.
2. Use Disease Free Plants
Use healthy plants, although symptoms may not be apparent at the time of field setting. If tips were infected, Phytophthora can show up in the plug plants and these plants should be culled or treated prior to field setting. Non-symptomatic plants may harbor the pathogen. In plug production facilities, plants should be on raised benches or on well drained gravel beds to prevent water movement from tray to tray and spread of the pathogen. Avoid highly susceptible varieties such as ‘Sweet Charlie’ on sites with a history of Phytophthora crown rot.
3. Monitor and Husbandry
Use proper irrigation practices; do not overwater as inoculum is spread by runoff. Do not use water for over-head (field) or mist irrigation (plug facilities) from surface ponds that may harbor the pathogen. If this is unavoidable, contact local extension services for information on reducing risk of Phytophthora problems from surface irrigation sources.
Chemical Control
Mefenoxam (Ridomil Gold) and metalaxyl (various formulations) have proven effective when applied through the drip irrigation. These fungicides are best applied in the fall soon after planting and when overhead watering for plant establishment is complete. In most cases, an early spring application is also useful when rapid new plant growth starts. The fall and spring timing coincide with rapid root growth. Additional applications can be made in the spring in fields with high disease pressure. The phosphonates do not have the same efficacy as mefenoxam/metalaxyl but are very useful during the plug production phase where problems exist, applied as a foliar spray in fields where strawberry roots are damaged, and in fields where mefenoxam/metalaxyl resistant Phytophthora strains have been documented (this has only occurred once in the Southeast). For more information on timing and use rates, see our Strawberry IPM Guide at for current recommendations.
Pathogen
Phytophthora Crown Rot (Phytophthora cactorum (Lebert and Cohn) J. Schröt.)
Phytophthora cactorum is a soil inhabiting Oomycete plant pathogen. P. cactorum has coenocytic hyphae and forms a white, loosely matted colony in culture (Erwin and Ribeiro, 1996). Oospores of P. cactorum are spherical (24-30 µm), have a smooth, two layered wall, and are produced after the fusion of an antheridium and oogonium. Antheridia typically are paragynous and usually attached to the oogonium near the oogonial stalk. P. cactorum produces caducous, pear-shaped sporangia (50-60 x 35-40 µm), each on a short (< 4 μm) pedicel. Sporangia usually are terminal and have prominent papillae (Ellis and Madden, 1998; Erwin and Ribeiro, 1996). P. cactorum is a homothallic species, meaning oospores can be found within single isolate cultures (Erwin and Ribeiro, 1996; Hudler, 2013).
Crown rot seems to be induced by a distinct pathotype of P. cactorum because only isolates from diseased strawberry crowns were pathogenic to strawberry in inoculation experiments (Seemüller, 1998).
Diagnostic Procedures
P. cactorum can be baited from soil and isolated from the root or crown tissue of infected plants using selective media. Most often P. cactorum is isolated using PPP or PARP and cultured on CMA or V8A. If baiting soil for P. cactorum it is important to dry soil samples for a time and then rehydrate them prior to baiting (Jeffers and Aldwinckle, 1987). Surface disinfesting crowns before plating may enhance isolation of P. cactorum. The pleurotic oospores of P. cactorum may also be present in crown tissue and are commonly present in black roots immediately adjacent to the infected crown areas (Figure SS-4).
A distinctive characteristic of this pathogen compared to other Phytophthora species is the caducous nature of the sporangia. Most isolates of P. cactorum readily fruit on plant tissue and in culture, however, if sporangia are absent, formation can be induced by immersing agar plugs from an actively growing culture in 1.5% non-sterile soil extract solution (NSSES) and incubating at room temperature under constant fluorescent light for 12 to 24 hours (Jeffers and Aldwinckle, 1987).
Phytophthora crown rot is hard to discern from anthracnose crown rot. A diagnostic key is provided (Figure DP-1). Serology assays such as immunostrips and other assays are commercially available.
The most conclusive means of identifying an isolate of Phytophthora is through molecular techniques. The ITS 1 and 2 regions of the rDNA and the cox 1 and 2 regions of mitochondrial DNA can be amplified using PCR, purified, and then sequenced. Sequences can then be compared to those of verified Phytophthora species recorded in databases like Phytophthora id and genbank.
References
Ellis, M. A., and Madden, L. V. 1998. Leather rot. Pp. 33-35 in: Compendium of Strawberry Diseases, 2nd edition, Maas, J. L. (edt.). APS Press. St. Paul, MN.
Erwin, D. C., and Ribeiro, O. K. 1996. Phytophthora Diseases Worldwide. American Phytopathological Society, St. Paul, MN.
Hudler, G. W. 2013. Phytophthora cactorum. Forest Phytophthoras 3(1). doi:10.5399/osu/fp.3.1.3396
Jeffers, S. N., and Aldwinckle, H. S. 1987. Enhancing detection of Phytophthora cactorum in naturally infested soil. Phytopathology 77:1475-1482.
Seemüller, E. 1998. Crown rot. Pp. 50-51 in: Compendium of Strawberry Diseases, 2nd edition, Maas, J. L. (edt.). APS Press. St. Paul, MN.